this is AMAZING….
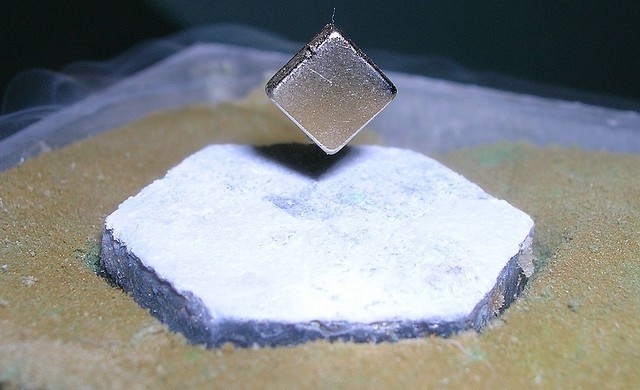
Superconductors may be instrumental in the future of transportation. Basically they are materials which can conduct electricity with just about zero resistance once they go below a particular temperature.
It is that “almost frictionless” state that is the key to their transportation capabilities. Now, they may have discovered properties of incredible performance from an unsuspecting compound:
Hydrogen sulfide — the compound responsible for the smell of rotten eggs — conducts electricity with zero resistance at a record high temperature of 203 kelvin (–70 °C), reports a paper published today in Nature1.
The first results of the work, which represents a historic step towards finding a room-temperature superconductor, were released on the arXiv preprint server in December2 and followed up by more in June3. They have already sparked a wave of excitement within the research community.
Do you think they will succeed in making a hyperloop with superconductors or perhaps a different application?
Or perhaps another project will be the first in the states to show off their incredible properties.
Let’s check out what happens when you mix ferrofluids and superconductors on page 2
I just realize something, they will never make such a discovery at room temperature. It is impossible. It does not exist anywhere in nature. Show me the source.
Forgive my ignorance on the matter. I cannot remember the scientific name of the device constructed but someone has created a way to cool down sodium atom to just above the point of absolute zero. I would like to see the results of using such frigid temperatures on superconductive materials. Also this is achieved by heating the sodium to 700 degrees then using lasers to cool it down. A female scientist made a way to slow down the speed of a laser to way below the speed of light. I wonder using such device to slow the laserlyte down will have an effect on the sodium atom.